Authors: Raktim Sen, Alain Goeppert, Sayan Kar, G. K. Surya Prakash*Loker Hydrocarbon Research Institute and Department of Chemistry, University of Southern California, Los Angeles, California, United States.
Full citation: Sen, R.; Goeppert, A.; Kar, S.; Prakash, G. K. S., Hydroxide Based Integrated CO2 Capture from Air and Conversion to Methanol. Journal of the American Chemical Society 2020, 142, 4544-4549.
Over the past two centuries, surging greenhouse gas emissions have led to an enormous rise in atmospheric CO2 levels, causing a significant increase in the average global temperature and ocean acidification. Humankind is now releasing every year > 36 billion tons of CO2 to the environment! Among various solutions explored to mitigate this problem, direct air capture (DAC) of CO2 is highly promising. DAC has significant advantages. It enables the point of capture to be independent from the emission sources. It also targets CO2 emissions from dispersed point sources, which are responsible for about half of total anthropogenic emissions but would otherwise be difficult to address. Furthermore, utilization of the captured CO2 (Carbon Capture and Utilization, CCU) as a carbon source for various chemical feedstocks and polymeric materials is very promising. In this context, CO2 is combined with hydrogen obtained by electrolysis of water using renewable energy sources such as solar and wind. Among various CO2 hydrogenation products, methanol is one of the most attractive and versatile alcohol. Methanol is a feedstock for a multitude of chemicals and products, already used on a scale of over 100 billion liters per year. Additionally, methanol, which is a liquid with a boiling point of 64.7 °C, is a superior fuel for internal combustion engines and fuel cells, as well as a convenient high density liquid hydrogen carrier. Methanol can be converted to ethylene and propylene through the MTO (methanol to olefins) process. Hence, the production of renewable methanol and the associated methanol economy holds significant potential to replace current fossil fuel-based economies.
Following the legacy of their late colleague Nobel Laureate George Olah, a major proponent of the methanol economy, G. K. Surya Prakash, professor and director at Loker Hydrocarbon Institute and his team including senior research scientist, Alain Goeppert have successfully combined the two steps of CO2 capture and hydrogenation to methanol into a seamless reaction sequence to transform the greenhouse gas into its combustible cousin. Until now, the integrated capture and conversion processes relied on organic bases called amines to capture CO2. While amines are easily regenerable, they are prone to oxidative degradation and suffer from notable toxicity and volatility. Hence, in order to improve the system, Prakash and his team turned to inorganic bases like sodium/potassium hydroxides which are superior CO2 capturing agents forming their respective bicarbonates/carbonates. Conventionally, however, recycling of the base by thermally decomposing bicarbonates/carbonates requires extreme temperatures above 700 °C making the process highly energy intensive. In initial efforts by the group, CO2 captured from air by a solution of hydroxide in water was converted to formate salts. However; further hydrogenation to methanol was ineffective.
In their current study, the authors discovered that switching water with alcohols like ethylene glycol as a reaction medium could mediate effectively the process to methanol. When air was bubbled through potassium hydroxide dissolved in ethylene glycol and the CO2-loaded solution subsequently hydrogenated in the presence of H2 and a metal catalyst, complete conversion to methanol was observed at 140 °C. Moreover, regeneration of the hydroxide base occurred at mild temperatures of 100-140 °C. Notably, a fraction of the base was deactivated in an unwanted side reaction. Currently, the researchers are aiming to minimize the side reactions to efficiently recycle the potassium hydroxide. This system offers easy integration of existing hydroxide-based CO2 scrubbing industries to utilize the captured CO2 to produce methanol, paving a way for a sustainable and carbon neutral future. As Prakash says, “All living things are primarily made of carbon and therefore carbon has to be managed. Humankind requires carbon based fuels and chemical feedstocks currently obtained from fossil fuels. Renewable methanol can easily replace fossil fuels without requiring major changes to our current chemical and transportation infrastructures. If CO2 is a one-carbon problem, then methanol is a one-carbon solution.”
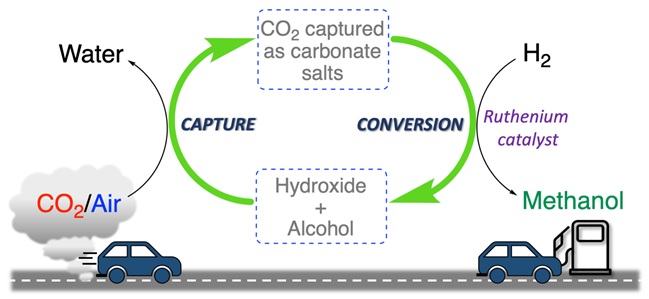
The study authored by Prakash, Goeppert and graduate assistants Raktim Sen and Sayan Kar was recently published in Journal of the American Chemical Society (DOI: 10.1021/jacs.9b12711) and also featured in C&E News (https://cen.acs.org/content/cen/articles/98/i10/One-pot-process-converts-CO2.html).
